Abstract
INTRODUCTION: Venetoclax (VEN), a BCL-2 specific inhibitor, used in combination with azacitidine (AZA) in patients with newly-diagnosed acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy treatment, has shown high response rate and overall survival compared with AZA monotherapy (Di Nardo et al. N Engl J Med 2020; 383:617-629). For patients failing frontline, VEN-based salvage therapy prognosis appears very poor, although a subgroup of patients might benefit. Moreover, recently, had been published the European LeukemiaNet 2022 recommendations and classification in AML (Döhner et al. Blood 2022).
AIMS: We performed a retrospective study of patients with relapsed/refractory (R/R) AML receiving salvage treatment with VEN combinations in the Catalan Institute of Oncology (ICO) in order to determine the efficacy and safety of the combination. We also explored the probability of response according to the ELN 2022 risk classification by genetics.
METHODS: We analyze 60 patients with R/R AML at 4 hospitals belonging to ICO in Spain, treated with VEN (400mg/24h or 600mg/24h; with recommended 3-day ramp-up to target dose) in combination with hypomethylating (HMA) agents (AZA 75mg/m2 7/28 days or Decitabine (DEC) 20mg/m2 5/28 days) or low-dose cytarabine (LDAC) (20mg/m2 10/28 days) from May 2019 until December 2021. Event was defined as death, refractoriness to treatment or progressive disease.
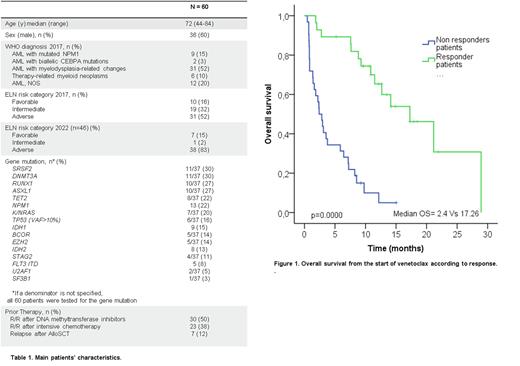
RESULTS: Characteristics of our cohort are described in table 1. Thirty-seven (62%) patients had next-generation sequencing (NGS) panel study at diagnosis and/or at the time of R/R disease. 31 (52%) patients had high-risk AML according to ELN 2017 classification, and 38 of 46 (83%) patients had high-risk AML according to ELN 2022 classification. Six of 37 patients (15%) had TP53 mutation at a variant allele fraction (VAF) of at least 10%. Seven (19%) patients were reclassified as high-risk AML according to ELN 2022 classification. NGS panel study didn't show high risk mutations in 10 patients. According to ELN 2022 classification, 8, 11 and 8 patients, presented 1, 2 or ≥ 3 high risk gene mutations, respectively. Fourteen (23%) patients received VEN + DEC, 34 (57%) VEN + AZA, and 12 (20%) VEN + LDAC. The median number of cycles received was 3 (range 1-28), with a median of one cycle to achieve the best response (range: 1-4). Early mortality in the first 30 days was 15% (9 patients), 5 due to progression disease, 2 due to infection and 2 due to clinical worsening. Overall response rate after first cycle [Complete response (CR) + complete response without hematological recovery (CRi) and partial response (PR)] was 58%. Three patients were treated in a molecular relapse (MRD positive status), and all of them achieved molecular response. Six of 19 fit young patients (32%) achieved CR and received an allogeneic stem cell transplantation (alloSCT). The median event-free survival (EFS) and overall survival (OS) was 4.03 months and 7.63 months, respectively. No significant differences of EFS and OS were found according to ELN 2022 risk classification, mutational profile or TP53 status. Response to treatment after 3-4 cycles, discriminate two groups of patients with an OS of 17.26 months in those patients who achieved CR or PR vs 2.4 months in non-responders (p=0.000). Patients relapsed after alloSCT (7 patients) presented a poor outcome (median OS was 1.6 months).
SUMMARY/CONCLUSIONS: Our study showed that real-world experience of treating patients with R/R AML with VEN + HMA or LDAC is feasible as salvage treatment in patients relapsed to HMA and as bridge therapy to alloSCT with a rapid response rate. No significant differences of response were found according to ELN 2022 risk classification. Rapid responses shown with the combination, allow us to identify those patients who may benefit from this approach.
Disclosures
Sureda:TAKEDA: Consultancy, Honoraria, Speakers Bureau; NOVARTIS: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; ROCHE: Consultancy, Honoraria; SANOFI: Consultancy, Honoraria; MSD: Honoraria; BMS: Consultancy, Honoraria; GILEAD: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.